Products and R&D
Current
Products
EnVAX-A71

AimFlu-S(QIS)
2312.jpg)
Tetanus vaccine adsorbed suspension for injection
Speedy COVID-19 Ag Rapid Test
Approval Number: Case-Specific Approval of Disease Control for Manufacture No.1106803257
Speedy COVID-19 Ag Rapid Test is a rapid lateral flow immunoassay for the qualitative detection and diagnosis of SARS-CoV-2 directly from nasopharyngeal swab. The use of a Speedy COVID-19 Ag Rapid Test will enable to verify infection quickly, begin proper treatment and to initiate isolation precautions helping prevent further spread of infection.
More test procedures, please refer to:
Pipeline
Vaccines
Vaccine is a highly effective method to prevent infectious diseases such as pneumococcus, Norovirus, Dengue fever virus), and Middle East respiratory syndrome (MERS).
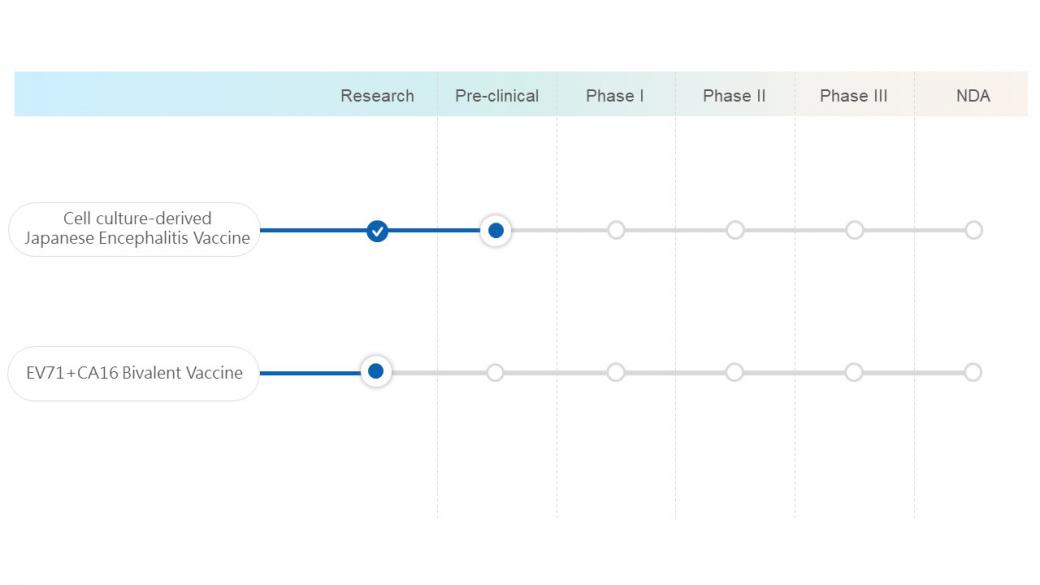
Cell culture-derived Japanese Encephalitis Vaccine
We plan to develop a vaccine for Japanese encephalitis virus (JEV) based on the cell culture platform. This product can replace the traditional Japanese encephalitis vaccine prepared from mouse brain. The Japanese encephalitis vaccine is a routine vaccination in Taiwan that is suitable for infants, preschool children, and adults with insufficient immune response.
Anti-RBD (SARS-CoV-2 Spike Protein) Monoclonal Antibody
It is a murine monoclonal antibody that can specifically recognize the receptor binding domain (RBD) of the SARS-CoV-2 spike protein, and it also has a good affinity to the receptor binding region (RBD) of SARS-CoV-2 variants recombinant protein, including beta strain (South Africa strain), gamma strain (Brazil strain) and delta strain (India strain). This antibody can be applied to develop several immunological test methods, such as, IVD kit, ELISA, western blot, immunostaining... etc.
EV71+CA16 Bivalent Vaccine
The hand-foot-mouth disease (HFMD) is mostly caused by Coxsackie A virus and enterovirus 71 (EV71). In recent years, frequent epidemics of enteroviruses were mostly caused by Coxsackie A virus, which contains 30 subtypes such as CA16, CA4, and CA6. Among them, CA16 is primary virus to cause HFMD. We are working with research institutions to develop EV71 plus CA16 bivalent vaccine, providing more extensive protection for children in Taiwan.

.jpg)